Abstract
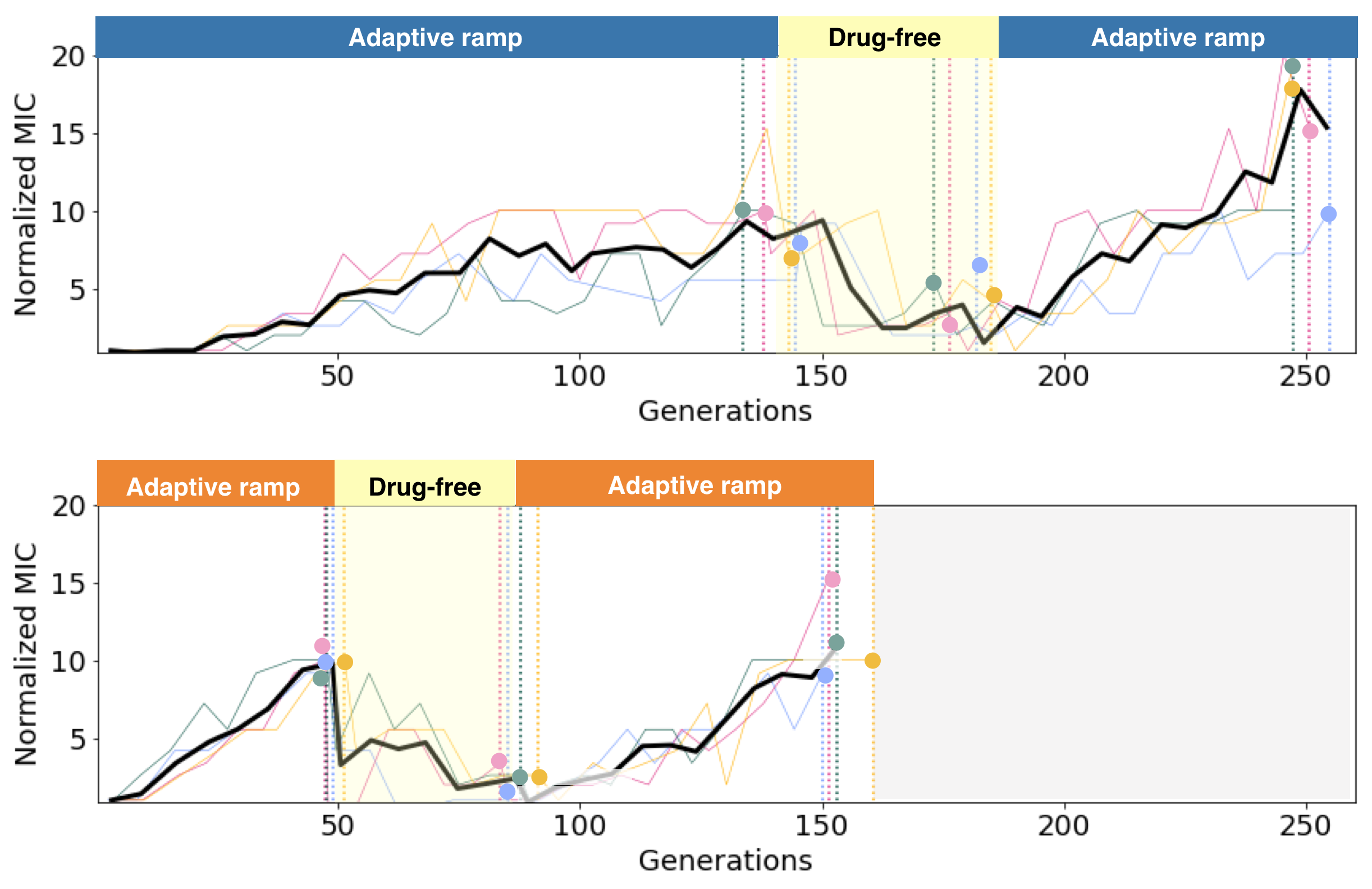
Bacterial adaptation to stressful environments often produces evolutionary constraints whereby increases in resistance are associated with reduced fitness in a different environment. The exploitation of this resistance-cost trade-off has been proposed as the basis of rational antimicrobial treatment strategies designed to limit the evolution of drug resistance in bacterial pathogens. Recent theoretical, laboratory, and clinical studies have shown that fluctuating selection can maintain drug efficacy and even restore drug susceptibility, but can also increase the rate of adaptation and promote cross-resistance to other antibiotics. In this paper, we combine mathematical modeling, experimental evolution, and whole-genome sequencing to follow evolutionary trajectories towards beta-lactam resistance under fluctuating selective conditions. Our experimental model system consists of eight populations of Escherichia coli K12 evolving in parallel to a serial dilution protocol designed to dynamically control the strength of selection for resistance. We implemented adaptive ramps with mild and strong selection, resulting in evolved populations with similar levels of resistance, but with different evolutionary dynamics and diverging genotypic profiles. We found that mutations that emerged under strong selection are unstable in the absence of selection, in contrast to resistance mutations previously selected in the mild selection regime that were stably maintained in drug-free environments and positively selected for when antibiotics were reintroduced. Altogether, our population dynamics model and the phenotypic and genomic analysis of the evolved populations show that the rate of resistance adaptation is contingent upon the strength of selection, but also on evolutionary constraints imposed by prior drug exposures.
 Download PDF [3.2 Mb]
Download PDF [3.2 Mb]
 Public Repository
Public Repository
Abstract
With plasmid-mediated antibiotic resistance thriving and threatening to become a serious public health problem, it is paramount to increase our understanding of the forces that enable the spread and maintenance of drug resistance genes encoded in mobile genetic elements. The relevance of plasmids as vehicles for the dissemination of antibiotic resistance genes, in addition to the extensive use of plasmid-derived vectors for biotechnological and industrial purposes, has promoted the in-depth study of the molecular mechanisms controlling multiple aspects of a plasmids' life cycle. This body of experimental work has been paralleled by the development of a wealth of mathematical models aimed at understanding the interplay between transmission, replication, and segregation, as well as their consequences in the ecological and evolutionary dynamics of plasmid-bearing bacterial populations. In this review, we discuss theoretical models of plasmid dynamics that span from the molecular mechanisms of plasmid partition and copy-number control occurring at a cellular level, to their consequences in the population dynamics of complex microbial communities. We conclude by discussing future directions for this exciting research topic.
 Download PDF [2758 kb]
Download PDF [2758 kb]
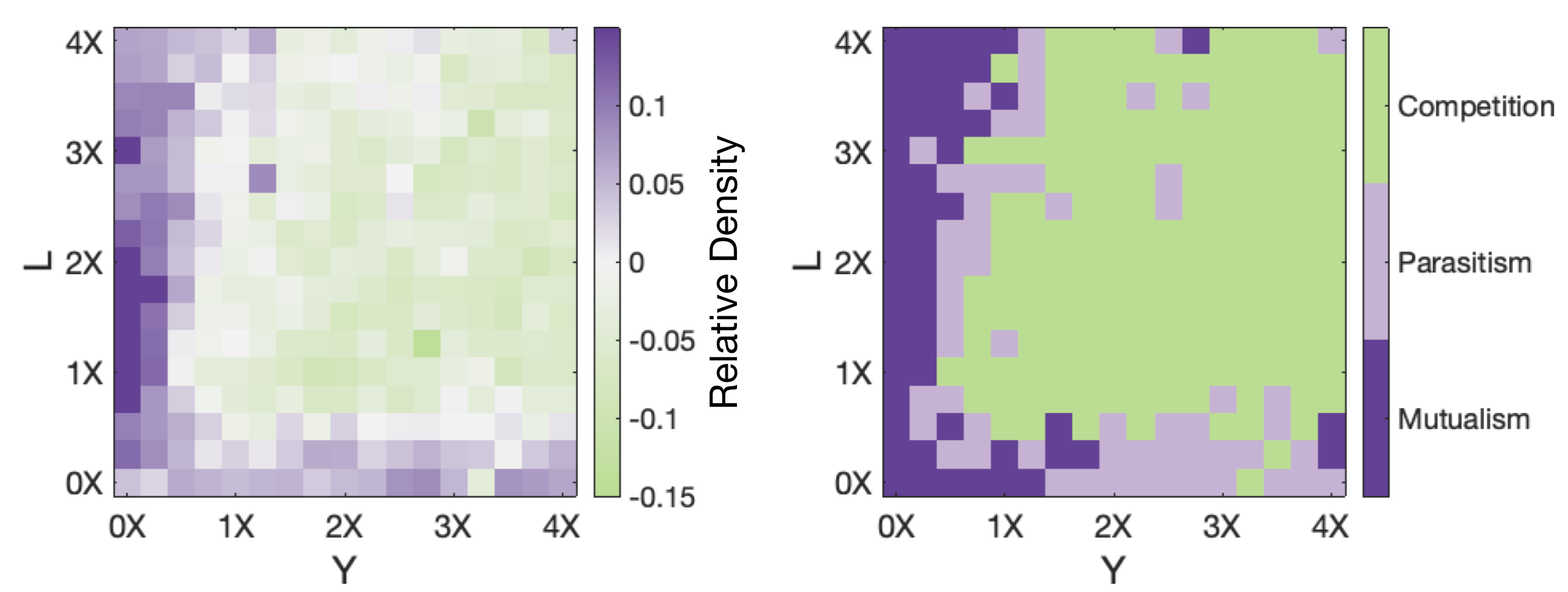
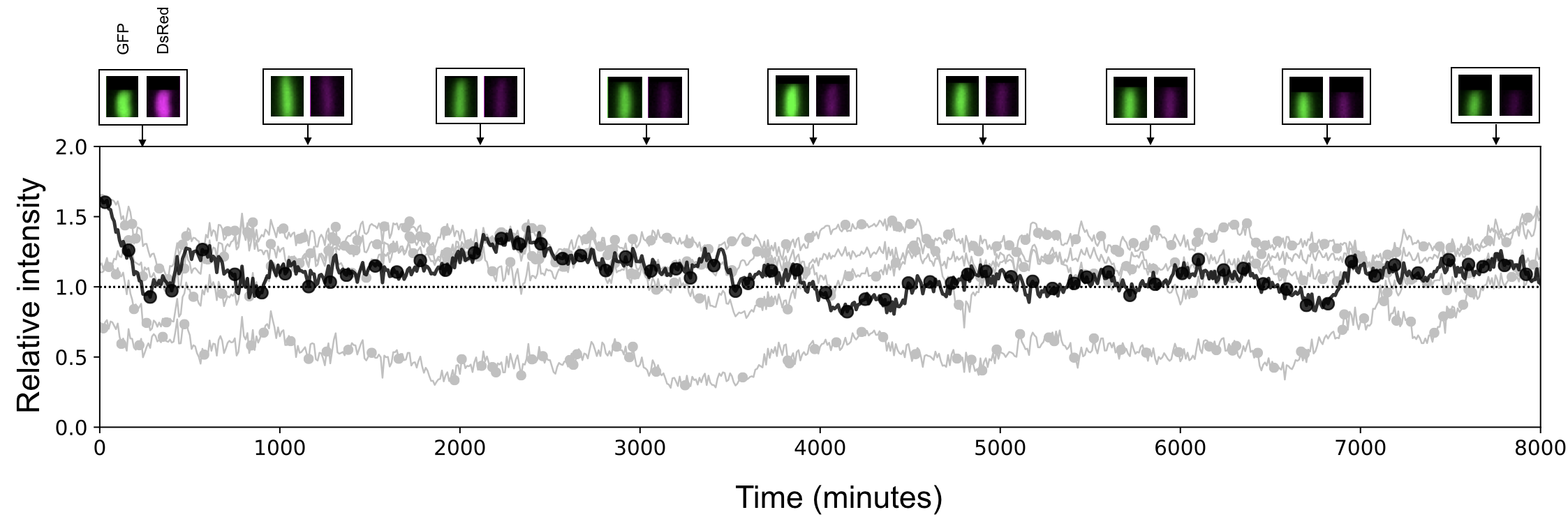
Abstract Multicopy plasmids play an important role in bacterial ecology and evolution by accelerating the rate of adaptation and providing a platform for rapid gene amplification and evolutionary rescue. Despite the relevance of plasmids in bacterial evolutionary dynamics, evaluating the population-level consequences of randomly segregating and replicating plasmids in individual cells remains a challenging problem, both in theory and experimentally. In recent years, technological advances in fluorescence microscopy and microfluidics have allowed studying temporal changes in gene expression by quantifying the fluorescent intensity of individual cells under controlled environmental conditions. In this paper, we will describe the manufacture, experimental setup, and data analysis pipeline of different microfluidic systems that can be used to study plasmid dynamics, both in single-cells and in populations. To illustrate the benefits and limitations of microfluidics to study multicopy plasmid dynamics, we will use an experimental model system consisting on Escherichia coli K12 carrying non-conjugative, multicopy plasmids (19 copies per cell, in average) encoding different fluorescent markers and beta-lactam resistance genes. First, we will use an image-based flow cytometer to estimate changes in the allele distribution of a heterogeneous population under different selection regimes. Then we will use a mothermachine microfluidic device to obtain time-series of fluorescent intensity of individual cells to argue that plasmid segregation and replication dynamics are inherently stochastic processes. Finally, using a microchemostat, we track thousands of cells in time to reconstruct bacterial lineages and evaluate the allele frequency distributions that emerge in response to a range of selective pressures.
 Download PDF [2758 kb]
Download PDF [2758 kb]
 Public Repository
Public Repository
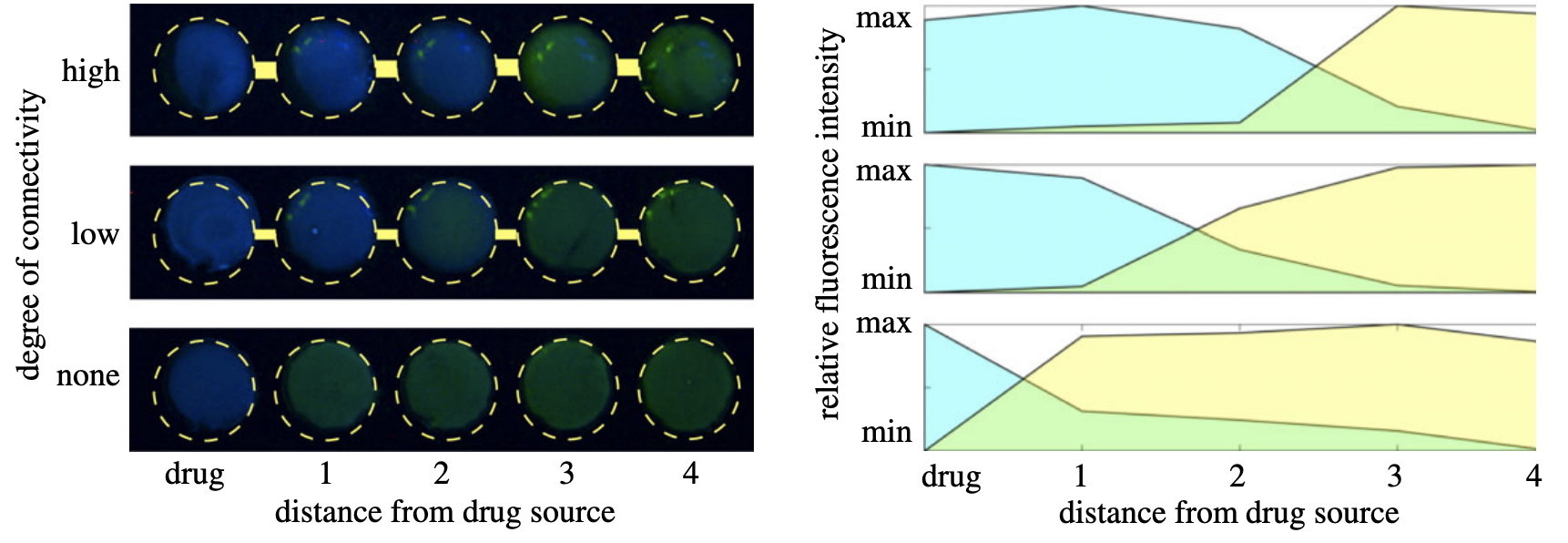
Abstract The current crisis of antimicrobial resistance in clinically relevant pathogens has highlighted our limited understanding of the ecological and evolutionary forces that drive drug resistance adaptation. For instance, although human tissues are highly heterogeneous, most of our mechanistic understanding about antibiotic resistance evolution is based on constant and well-mixed environmental conditions. A consequence of considering spatial heterogeneity is that, even if antibiotics are prescribed at high dosages, the penetration of drug molecules through tissues inevitably produces antibiotic gradients, exposing bacterial populations to a range of selective pressures and generating a dynamic fitness landscape that changes in space and time. In this paper, we will use a combination of mathematical modelling and computer simulations to study the population dynamics of susceptible and resistant strains competing for resources in a network of micro-environments with varying degrees of connectivity. Our main result is that highly connected environments increase diffusion of drug molecules, enabling resistant phenotypes to colonize a larger number of spatial locations. We validated this theoretical result by culturing fluorescently labelled Escherichia coli in 3D-printed devices that allow us to control the rate of diffusion of antibiotics between neighbouring compartments and quantify the spatio-temporal distribution of resistant and susceptible bacterial cells.
 Download PDF [737 kb]
Download PDF [737 kb]
Abstract Understanding the mechanisms governing innovation is a central element of evolutionary theory. Novel traits usually arise through mutations in existing genes, but trade-offs between new and ancestral protein functions are pervasive and constrain the evolution of innovation. Classical models posit that evolutionary innovation circumvents the constraints imposed by trade-offs through genetic amplifications, which provide functional redundancy. Bacterial multicopy plasmids provide a paradigmatic example of genetic amplification, yet their role in evolutionary innovation remains largely unexplored. Here, we reconstructed the evolution of a new trait encoded in a multicopy plasmid using TEM-1 beta-lactamase as a model system. Through a combination of theory and experimentation, we show that multicopy plasmids promote the coexistence of ancestral and novel traits for dozens of generations, allowing bacteria to escape the evolutionary constraints imposed by trade-offs. Our results suggest that multicopy plasmids are excellent platforms for evolutionary innovation, contributing to explain their extreme abundance in bacteria.
 Download PDF [5143 kb]
Download PDF [5143 kb]
Abstract We need to find ways of enhancing the potency of existing antibiotics, and, with this in mind, we begin with an unusual question: how low can antibiotic dosages be and yet bacterial clearance still be observed? Seeking to optimise the simultaneous use of two antibiotics, we use the minimal dose at which clearance is observed in an in vitro experimental model of antibiotic treatment as a criterion to distinguish the best and worst treatments of a bacterium, Escherichia coli. Our aim is to compare a combination treatment consisting of two synergistic antibiotics to so-called sequential treatments in which the choice of antibiotic to administer can change with each round of treatment. Using mathematical predictions validated by the E. coli treatment model, we show that clearance of the bacterium can be achieved using sequential treatments at antibiotic dosages so low that the equivalent two-drug combination treatments are ineffective. Seeking to treat the bacterium in testing circumstances, we purposefully study an E. coli strain that has a multidrug pump encoded in its chromosome that effluxes both antibiotics. Genomic amplifications that increase the number of pumps expressed per cell can cause the failure of high-dose combination treatments, yet, as we show, sequentially treated populations can still collapse. However, dual resistance due to the pump means that the antibiotics must be carefully deployed and not all sublethal sequential treatments succeed. A screen of 136 96-h-long sequential treatments determined five of these that could clear the bacterium at sublethal dosages in all replicate populations, even though none had done so by 24 h. These successes can be attributed to a collateral sensitivity whereby cross-resistance due to the duplicated pump proves insufficient to stop a reduction in E. coli growth rate following drug exchanges, a reduction that proves large enough for appropriately chosen drug switches to clear the bacterium.
 Download PDF [1156 kb]
Download PDF [1156 kb]